I. General Introdution
Corrosion problem is one of the main causes of damage to structures (pipes, tanks ...). The corrosive environment includes the internal and external environment. The phenomenon of corrosion is the electrochemical reaction of metal or alloys with the surrounding environment, causing a part of the metal or alloy. A part of the metal tends to become anode and the other becomes a cathode. At anode, the metal is dissolved and the corrosion occurs. One of the corrosive places that occur is an electrochemical anti -corrosion method (Cathode protection method)
This method has 2 ways:
- Use sacrificial anodes.
- Use of impressed current anodes.
II. Electrochemical corrosion system used to sacrificial anodes
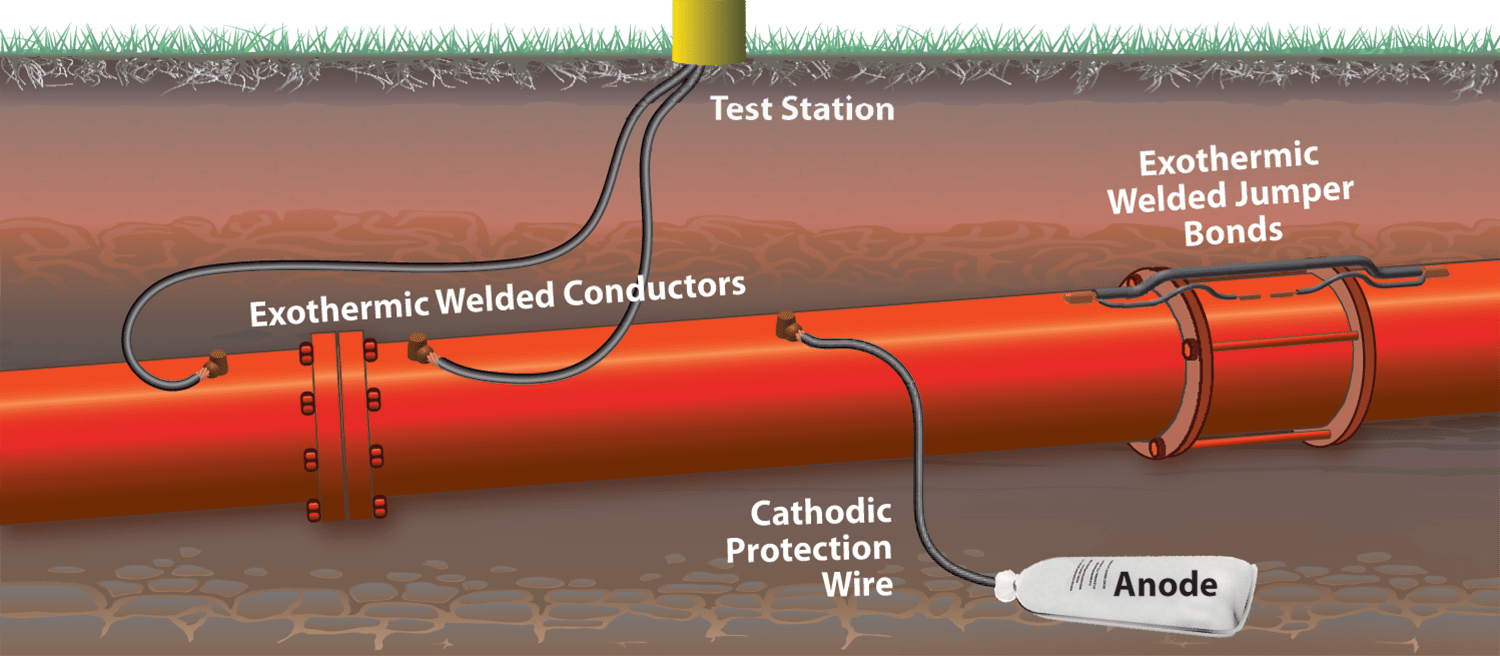
- A method of using an anode (metal or alloy) preceded in the electrolytic series (compared to a metal or alloy of a pipe) to generate a potential that prevents an electrochemical reaction from occurring in metals to be protected.
- The protection system consists of 2 main parts:
- The structure needs to be protected: pipes, tanks ...
- Anode protection.
- Anode provides protection are usually made by Al, Mg,Zn alloys.
- Principle diagram:

III. Electrochemical corrosion protection system using impressed current anodes
- A method of using an external current anode that establishes the voltage difference by an external electromagnetic power supply device. During the electrochemical corrosion process, oxidation reactions at the anode and reactions at the cathode occur.
- The protection system consists of 3 main parts:
+ Structures need to be protected: pipes, tanks ...
+ Anode protection: graphite, iron – silicon ...
+ External current: 1-phase or 3-phase power can be used via MBA rectifier to 1-way current.
- Principle diagram:

IV. Advantages of corrosion resistance methods by impressed current
- Provide a large area of protection.
- Can adjust current and voltage to protect in accordance with changes in the environment and building.
- Can be applied to buildings without coating or poor coating.
 V.T.E.C.H ELECTRICAL TECHNOLOGY CO., LTD
V.T.E.C.H ELECTRICAL TECHNOLOGY CO., LTD